Balbharti Maharashtra State Board Class 9 Science Solutions Chapter 13 Carbon: An Important Element Notes, Textbook Exercise Important Questions, and Answers.
Maharashtra Board Class 9 Science Solutions Chapter 13 Carbon: An Important Element
Q.1. Fill in the blank and rewrite the completed statements: 16
1. An allotrope of carbon that is used to make ornaments is ……………
Ans An allotrope of carbon that is used to make ornaments is Diamond.
2. Compounds obtained directly or indirectly from plants and animals are called ……………
Ans Compounds obtained directly or indirectly from plants and animals are called Organic compounds.
3. A carbon atom forms a …………… bond with other atoms. In this bond the two atoms electrons.
Ans A carbon atom forms a covalent bond with other atoms. In this bond the two atoms share electrons.
4. The bond formed by sharing of electrons is called bond.
Ans The bond formed by sharing of electrons is called Covalent bond.
5. In graphite crystal, one layer of graphite is called ……………
Ans In graphite crystal, one layer of graphite is called Graphene.
6. Molecular formula of methane is ……………
Ans Molecular formula of methane is CH4.
7. The electronic configuration of carbon is ……………
Ans The electronic configuration of carbon is 2,4.
8. An allotrope of carbon that is rarely found in nature is ……………
Ans An allotrope of carbon that is rarely found in nature is Fullerene.
9. All the carbon bonds in a saturated hydrocarbon electrons.
Ans All the carbon bonds in a saturated hydrocarbon share electrons.
10.The production of biogas is an/a process.
Ans The production of biogas is an/a an anaerobic process.
11.The compounds formed from only carbon and hydrogen are called ……………
Ans The compounds formed from only carbon and hydrogen are called Hydrocarbons.
12. is the essential element in all the organic compounds.
Ans Carbon is the essential element in all the organic compounds.
13 .Atomic number of carbon is ……………
Ans Atomic number of carbon is 6.
14.The element hydrogen is present in organic compound.
Ans The element hydrogen is present in all organic compound.
15.At least one carbon bond in an unsaturated hydrocarbon is ……………
Ans At least one carbon bond in an unsaturated hydrocarbon is multiple.
16. The organic compound having double or triple bond in them is termed as ……………
Ans The organic compound having double or triple bond in them is termed as Unsaturated hydrocarbon.
Q. 2. Find the odd one out : 7
1. Coal, Petroleum, Natural Gas, Fats
Ans Fats – are an odd one out as it is a carbonaceous nutrient while the rest are fossil fuel.
2. Methane, Ethene, Propane, Butane
Ans Ethene – is the odd one out as it an unsaturated hydrocarbon while rest all are saturated hydrocarbon.
3. Lubricants, Paints, Ornaments, Electrodes
Ans Ornaments – are the odd one out as it is made from diamonds while rest is made from graphite.
4. Diamond, Graphite, Fullerene, Methane
Ans Methane – is an odd one out as it a marsh gas while rest are allotropes of carbon.
5. Peat, Lignite, Charcoal, Bituminous
Ans Charcoal – is the odd one out rest are all types of coal
6. Cotton, Wool, Silk, Protein
Ans Protein – is the odd one out as it is a carbonaceous nutrient while the rest are natural fibers.
7. CH4, C2H6, C3H8, C2H4
Ans C2H4 – is the odd one out as it is an unsaturated hydrocarbon while rest are examples of saturated hydrocarbon.
Q. 3. Find co-related terms 5
1. Fossil fuels: Petroleum : : Carbohydrates.
Ans Fossil fuels: Petroleum : : Carbonaceous nutrients : Carbohydrates.
2. Ethane : CH3–CH3 :: Ethene : ……………
Ans Ethane : CH3–CH3 :: Ethene : CH2=CH2.
3. Coal : Fuel in thermal power plants:: Coke : ……………
Ans Coal : Fuel in thermal power plants:: Coke : Domestic fuel.
4. Diamond : Tetragonal :: Graphite: ……………
Ans Diamond : Tetragonal :: Graphite: Hexagonal.
5. Propane : Single bond :: Propyne : ……………
Ans Propane : Single bond :: Propyne : Triple bond.
Q. 4. Match the pair 3
| Column A | Column B | Answer |
|
a. Arc lamps | c. Eye surgery |
| 2. Graphite | b. Insulator | a. Arc lamps |
| c. Eye surgery |
| Column A | Column B | Answer | |
| i. | Fossil fuel | a. Cotton | Natural gas |
| ii. | Carbonaceous nutrients | b. Natural gas | Fats |
| . c. Fats |
Q. 5. State True or False 15
1. Hydrocarbons with double bonds are called as saturated hydrocarbon.
Ans False – Hydrocarbons with double bonds are called as unsaturated hydrocarbon.
2. Covalent compounds are soluble in organic solvents.
Ans Covalent compounds are soluble in organic solvents – True
3. The production of biogas is an aerobic process.
Ans False – The production of biogas is an anaerobic process.
4. Graphite is used in making lubricants.
Ans Graphite is used in making lubricants – True
5. Coke is used as an oxidizing agent.
Ans False – Coke is used as a reducing agent.
6. Anthracite is known as the pure form of the coal.
Ans Anthracite is known as the pure form of the coal – True
7. Diamond is a good conductor of electricity.
Ans False – Diamond is a bad conductor of electricity./ Graphite is a good conductor of electricity.
8. Fullerenes are used as a catalyst in water purification.
Ans Fullerenes are used as a catalyst in water purification – True
9. Methane is a colorless gas.
Ans Methane is a colorless gas – True
10. Carbon dioxide is used to make aerated drinks.
Ans Carbon dioxide is used to make aerated drinks – True
11. Elements having same physical properties and different chemical properties are called allotropes.
Ans False – Elements having same chemical properties and different physical properties are called allotropes.
12. Methane is used for production of organic compounds such as ethanol.
Ans Methane is used for production of organic compounds such as ethanol – True
13. Liquified Carbon dioxide is used to remove caffeine from coffee.
Ans Liquified Carbon dioxide is used to remove caffeine from coffee – True
14. Compounds obtained from minerals are called inorganic compounds.
Ans Compounds obtained from minerals are called inorganic compounds – True
15. The density of methane is less than that of water.
Ans The density of methane is less than that of water – True
Q. 6. Name the following: 15
1. The substance used to remove caffeine from coffee.
Ans Liquefied Carbon dioxide
2. The type of coal that contains 70 to 90% of carbon.
Ans Bituminous coal
4. An allotrope of carbon that is used in arc lamps that gives a very bright light.
Ans Graphite
4. Name the chemicals used in the regular fire extinguishers.
Ans Sodium bicarbonate and dilute sulphuric acid
5. An allotrope of carbon that is used for making windows in artificial satellites.
Ans Diamond
6. Elements with the same chemical properties but different physical properties.
Ans Allotropes
7. An allotrope of carbon that is used as a catalyst in water purification.
Ans Fullerene
8. Hydrocarbons have at least one multiple bond.
Ans Unsaturated hydrocarbons
9. Which of the following organic compounds is unsaturated? (CH4, C3H6)
Ans C3H6 is an unsaturated organic compound where as CH4 is a saturated hydrocarbon.
10 . Name the organic solvents in which Fullerene is soluble.
Ans Carbon disulfide and Chlorobenzene.
11. The compounds obtained from minerals.
Ans Inorganic compounds
14. . The pure form of coal contains about 95% of carbon.
Ans Anthracite
13. Name any two organic compounds prepared from methane.
Ans Ethanol, methyl chloride, methylene chloride, and acetylene. (any two)
14. The type of coal that contains a high proportion of water and less than 60% of carbon.
Ans Peat
15. The compounds directly or indirectly obtained from plants.
Ans Organic compounds.
Q.7. Multiple Choice Questions: 12
1. Which of the following has a double bond?
a. hydrogen molecule b. Oxygen molecule
c. Nitrogen molecule d. Ammonia molecule
Ans Option b.
2. The property of carbon dioxide gas is that it …………….
a. supports combustion b. turns red litmus blue
c. turns blue litmus red d. pungent odors
Ans Option c.
3. coal contains the highest percentage of carbon.
a. Bituminous b. Peat c. Anthracite d. Lignite
Ans Option c.
4. How many single bonds are present in methane?
a. Four b. Five c. Six d. Three
Ans Option a.
5. Covalent compounds have …………….
A . High melting point b. Low melting point
c. very low melting point d. Very high melting point
Ans Option b.
6. Carbon dioxide gas is not used in …………….
a. Aerated drinks b. Fire extinguishers
c. Photosynthesis d. Glass cutting
Ans Option d.
7. Covalent compounds are generally insoluble in solvents.
a. universal b. inorganic c. organic d. acidic
Ans Option c.
8. How many carbon atoms are present in one molecule of ethanol?
a. One b. Two c. Three d. Four
Ans Option b.
9. Carbon has four valence electrons in its outermost shell, hence it is ……………
a. monovalent b. Bivalent c. Trivalent d. Tetravalent
Ans Option d.
10. Melting point of the diamond is ……………
a. 3700°C b. 3600°C c. 3500°C d. 3400°C
Ans Option c.
11. The molecular formula of Methane is …………….
a. C2H4
Ans Option d.
1. C2H6
2. C2H2
3. CH4
12. Which of the following has a triple bond?
a. hydrogen molecule b. Oxygen molecule
c. Nitrogen molecule d. Ammonia molecule
Ans Option c.
Q. 8. Chemical reaction with equation : 26
1. Carbon dioxide is passed through an aqueous solution of Sodium carbonate.
Ans When Carbon dioxide is passed through aqueous solution of Sodium carbonate, it forms Sodium bicarbonate.
Chemical reaction :
| Na2CO3 | + | CO2 | + | H2O | − − − − −− → | 2NaHCO3 |
| Sodium Carbonate | Carbon dioxide | Water | Sodium bicarbonate |
2. State the structural formula and electron dot model of Methane.
Ans :

3. Explain the following chemical reaction with balanced chemical reaction:- Coal is burnt in air.
Ans When coal is burnt in air, the carbon present in coal combines with oxygen present in air to form carbon dioxide gas.
| Chemical reaction: | C |
| Carbon |
| O2 | − − − − − → | CO2 ⇑ |
| Oxygen | Carbon dioxide |
4. Carbon dioxide gas is dissolved in water.
Ans Carbon dioxide is fairly soluble in water. It dissolves in water to form Carbonic acid.
Chemical reaction:
| CO2 | + | H2O | − − − − −− → | H2CO3 |
| Carbon dioxide | Water | Carbonic acid |
5. Sodium bicarbonate reacts with dilute Sulphuric acid.
Ans When sodium bicarbonate reacts with dilute Sulphuric acid, it forms Sodium sulphate , water and Carbon dioxide gas.
(Reaction in Fire extinguisher) Chemical reaction:
| 2NaHCO3 | + | H2SO4 | − − − − −− → | Na2SO4 | + | 2H2O | + | 2CO2 |
| dil.
Sulphuric acid |
||||||||
| Sodium Bicarbonate | Sodium sulphate | Water | Carbon dioxide | |||||
6. Complete the following chemical reaction:
i. 2NaOH +CO2 ….. → ![]()
![]()
ii. 2NaHCO3 + H2SO4 …..→ ![]() +
+![]() +
+![]()
Ans i. 2NaOH +CO2 → Na2CO3 + H2O
ii. 2NaHCO3+H2SO4……. → Na2SO4 + 2 H2O + 2CO2
7. Calcium carbonate reacts with dilute hydrochloric acid.
Ans When Calcium carbonate reacts with dilute hydrochloric acid it forms Calcium chloride, water and Carbon dioxide gas is evolved.
Chemical reaction:
| CaCO3 | + | 2HCl | − − − − −− → | CaCl2 | + | H2O | + | CO2 ⇑ |
| Calcium Carbonate | dil.
Hydrochloric |
|||||||
| Calcium Chloride | water | Carbon dioxide | ||||||
8. Methane is burnt in the air.
Ans Methane is highly inflammable and it burns completely by reacting with oxygen in the air with a bluish flame to form Carbon dioxide and water. (In this reaction 213kcal/mol of heat is given out)
Chemical reaction:
| CH4 | 2O2 | − − − − −− → | CO2 | + | 2H2O | + | Heat |
| Methane | Oxygen | Carbon dioxide | Water |
9. Methane reacts with chlorine gas. (Chlorination of methane)
Ans Methane and chlorine gas react with each other at the temperature of 250 to 400°C in presence of ultraviolet light to form Methyl chloride (Chloromethane) and hydrogen chloride. This reaction is called the Chlorination of methane.
Chemical reaction:
| Light
− − − − −− → |
||||||
| CH4 | + | Cl2 | CH3Cl | + | HCl | |
| Methane | Chlorine gas | Methyl chloride | Hydrogen chloride |
10. Production of Methane in biogas.
Ans In Biogas plant during the second stage, the methanogenic bacteria act on the organic acids to produce Methane gas and carbon dioxide gas.
(In the first stage, the microbes in the biogas act on the biodegradable complex organic compounds and produce organic acids)
Chemical reaction:
| CH3COOH | − − − − −− → | CH4 | + | CO2 ⇑ |
| Acetic acid (Organic acid) | Methane | Carbon dioxide |
11. Carbon dioxide is passed through freshly prepared lime water.
Ans When Carbon dioxide is passed through lime water, it forms water and a white precipitate of Calcium carbonate because of which lime water turns milky.
Chemical reaction:
+
Carbon dioxide
CO2
| Ca(OH)2 | − − − − −− → | CaCO3 | + | H2O |
| Calcium hydroxide | Calcium carbonate | water |
12. Complete the following chemical reaction:
1 .……………………………… + ……………………….. → CO2 + 2H2O + Heat
2. …………………… ……+ …………………………………→ CH3Cl + HCl
Ans 1. CH4 + 2O2 → CO2 + 2H2O + Heat
2. CH4+ Cl2 → CH3Cl + HCl
13. Complete the following chemical reaction:
i. 2NaHCO3+H2SO4 …..→ ………………….+…………… +…………….
ii.CaCO3. +2HCl …..→ ………………..+ H2O + CO2
Ans i. 2NaHCO3+H2SO4……. → Na2SO4 + 2 H2O + 2CO2
ii. CaCO3. +2HCl → CaCl2 + H2O + CO2
Q. 9. Write Short Notes 8
1. Fire extinguisher
Ans i. A Fire extinguisher contains sodium bicarbonate powder.
ii. There is also dilute sulphuric acid placed in a glass capsule.
iii. The capsule breaks on pressing the knob, the sulphuric acid comes in contact with the sodium bicarbonate and the two react chemically to release CO2.
iv. CO2 based fire extinguishers do not cause corrosion and are non-conductors of electricity. Therefore these are used when electrical and electronic equipment catches fire.
v. In Modern fire extinguishers liquid and solid CO2 is filled under pressure.
vi. On reducing the pressure it becomes gaseous and come out forcefully through the horn-like hose pipe.
2. Biogas plant
Ans i. Animal dung, dry leaves, wet garbage get decomposed by anaerobic microbes in a biogas plant. This produces methane gas also called biogas.
- Biogas is a very cheap fuel option which meets the demand for cooking gas. It is also used for production of electricity.
- Biogas is a fuel which is convenient to use, also very good manure is also produced as a side product of the process.
- Production of biogas is an anaerobic process. It takes place in two stages
- Production of acids
- Methane gas production
3. Allotropy.
Ans i. Some elements occur in nature in more than one form.
ii. These elements have same chemical properties but different physical properties. iii.This property of elements is called allotropy.
iv. Like carbon, sulphur and phosphorus also exhibit allotropy.
4. Coal and its types.
Ans i.. Coal is a fossil fuel. It contains carbon, hydrogen and oxygen. It also contains nitrogen, phosphorus and sulphur. It occurs in the solid state. It is of four types.
ii. Peat: Formation of peat is the first step in the formation of coal. It contains a high proportion of water and less than 60% of carbon.
iii. Lignite: Peat is transformed into lignite due to increased pressure and temperature inside the earth. It contains 60 to 70% of carbon. Lignite is the second step of the formation of coal.
iv. Bituminous coal: Bituminous coal was formed as the third step of formation of coal. It contains 70 to 90% of carbon.
v. Anthracite: Anthracite is known as the pure form of coal. This coal is hard and contains about 95% of carbon.
Q.10 .Attempt the following. 36
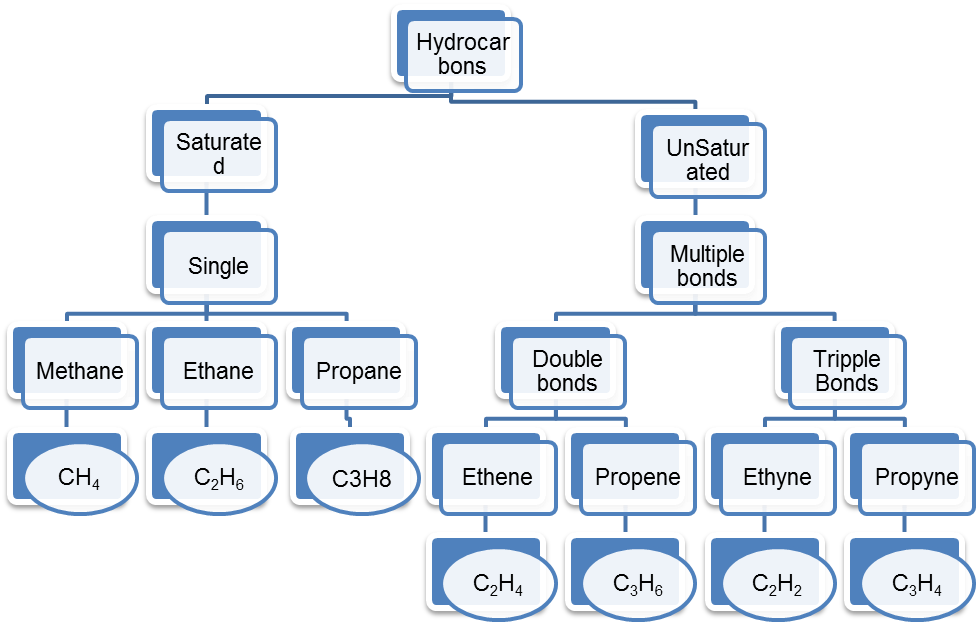
1. A hydrocarbon molecule contains 4 hydrogen atoms. Give its molecular formula, if it is an: (any 2)
i. alkane ii. alkene iii. alkyne
Ans i.. An alkane containing 4 hydrogen atoms in its molecule is Methane, CH4
ii. An alkene containing 4 hydrogen atoms in its molecule is Ethene, C2H4
iii. An alkane containing 4 hydrogen atoms in its molecule is Propyne, C3H4
2. Label the given diagram. The internal structure of Fire extinguisher.
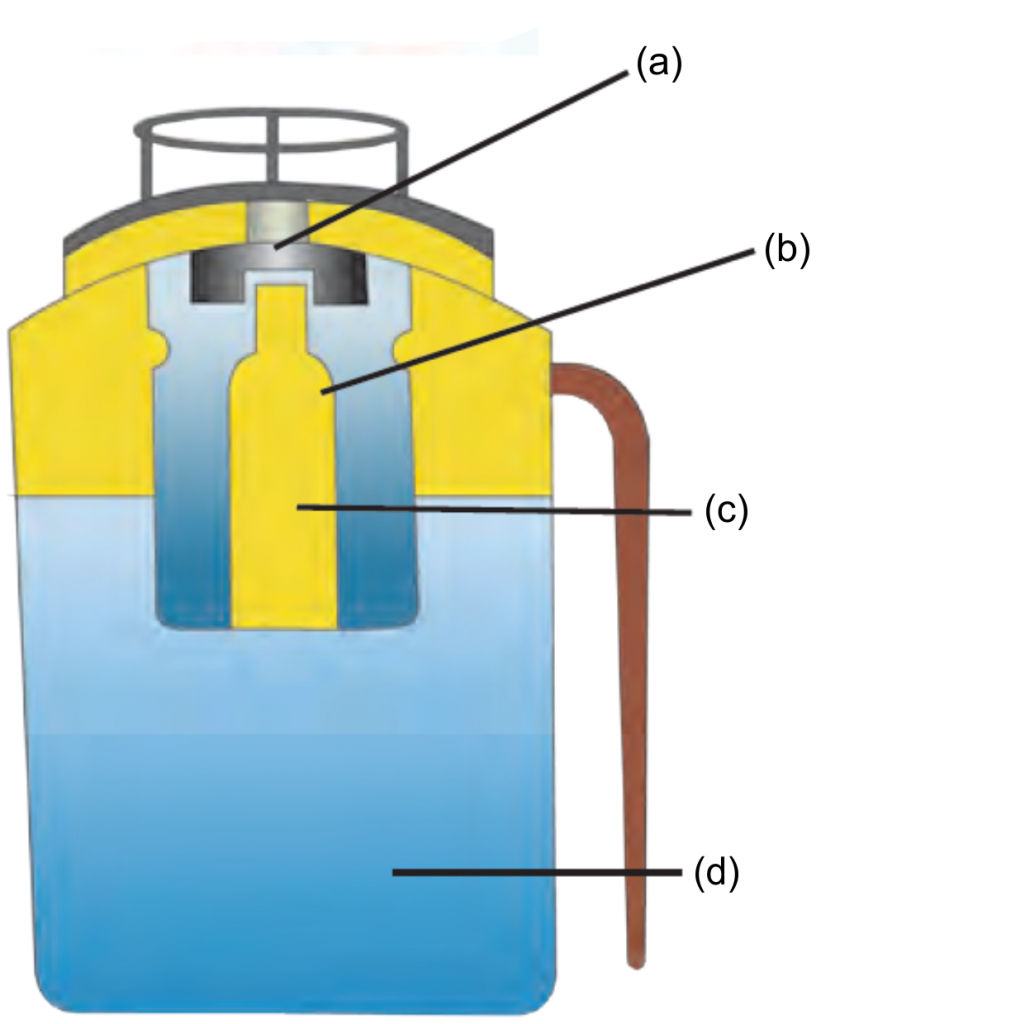
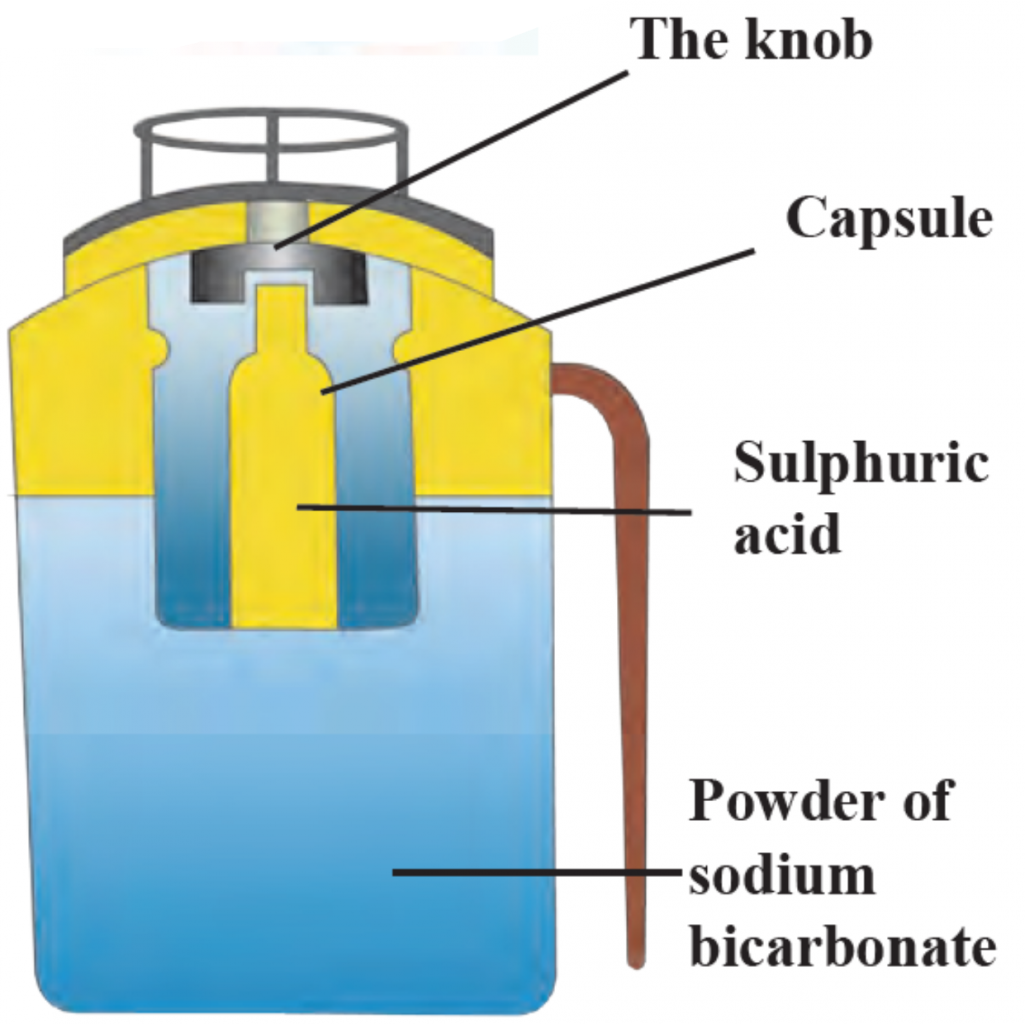 Ans
Ans
3. Classify the following:
(Coal, Carbohydrates, Petroleum, Cotton, natural gas, Fats, Wool)
| Fossil Fuels | Carbonaceous Nutrients | Natural Fibres |
| …………… | …………… | …………… |
Ans
| Fossil Fuels | Carbonaceous nutrients | Natural fibres |
| Coal, Petroleum, Natural gas | Carbohydrates, Fats | Cotton, Wool |
4. Give physical properties of Diamonds.
Ans The properties of Diamond are:
i. Brilliant and pure diamond is the hardest natural substance.
ii. The density of diamond is 3.5 g/cm3.
iii. The melting point of diamond is 3500°C.
iv. Diamond does not dissolve in any solvent.
v. Acids/Bases have no effect on diamond.
5. Complete the flow chart:
Ans
6. Solubility of Carbon.
Take 3 conical flasks and add cooking oil, water and kerosene in each of them respectively. Add a half spoonful of coal powder in each of the conical flasks and stir with the help of stirrer. Observe the mixture in the three conical flasks.

i. In which of the above solvents does the coal powder dissolve?
ii. What inference will you draw about the above solubility?
Ans i. Coal powder being organic in nature and being covalently bonded will dissolve in organic solvents like kerosene and oil but will be insoluble in water.
ii. Carbon is insoluble in water but soluble in organic solvents.
7. Give uses of Carbon dioxide in fire extinguisher.
Ans i. A Fire extinguisher contains sodium bicarbonate powder.
ii.Dilute sulphuric acid is placed in a glass capsule.
iii. The capsule breaks on pressing the knob, the sulphuric acid comes in contact with the sodium bicarbonate and the two react chemically to release CO2.
iv. CO2 based fire extinguishers do not cause corrosion and are non-conductors of electricity. Therefore these are used when electrical and electronic equipment catches fire.
v. CO2 based fire extinguishers are used to extinguish small scale fire.
vi. In Modern fire extinguishers liquid and solid CO2 is filled under pressure. On reducing the pressure it becomes gaseous and come out forcefully through the Horn-like hose pipe.
8. Give uses of allotropes of carbon : Coal
Ans Coal: The uses of coal are:
a. Coal is used as fuel in factories and homes.
b. Coal is used to obtain coke, coal gas and coal tar.
c. Coal is used in thermal power plants for generation of electricity.
9 Give uses of allotropes of carbon : Diamond
Ans Diamond: The uses of Diamond are:
a. Diamonds are used in glass cutting and rock drilling machines.
b. Diamonds are used in ornaments.
c. Diamonds knives are used in the eye surgery.
d. Diamond dust is used for polishing other diamonds.
e. Diamond is used to make windows giving protection from radiation in space and in artificial satellites.
10. Give physical properties of Fullerence.
Ans 1.. Fullerene, an allotrope of carbon, is rarely found in nature.
2 Molecules of fullerenes are found in the form of buckyballs and buckytubes.
3 Fullerenes are soluble in organic solvents such as carbon disulphide, chlorobenzene.
4 At a certain temperature fullerene exhibits superconductivity.
11. Label the given diagram
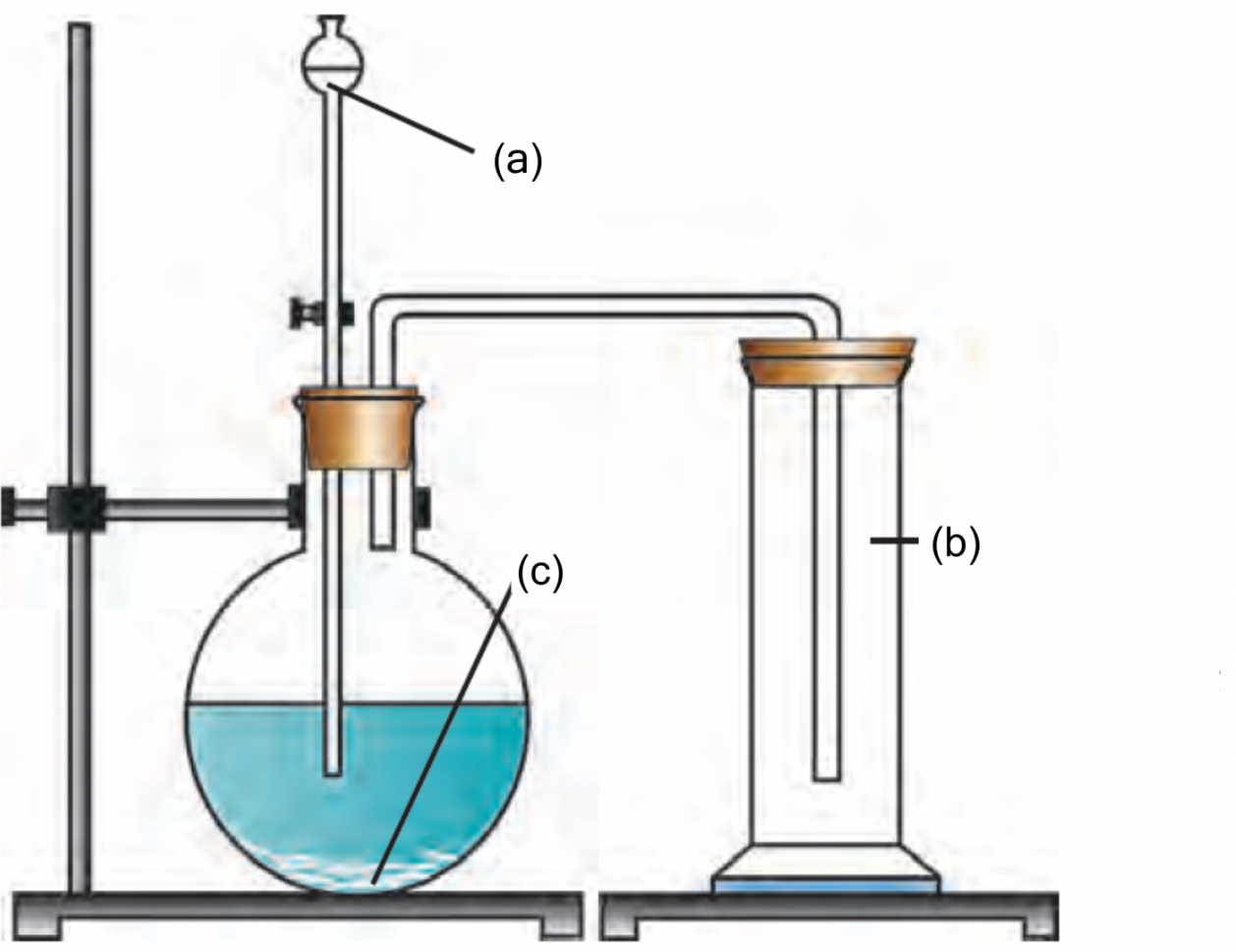 Preparation of Carbon dioxide:
Preparation of Carbon dioxide:
Ans
 .
.
12. Give the properties of Fullerene
Ans The properties of Fullerene are:
i. Molecules of fullerenes are found in the form of buckyballs and buckytubes.
ii. There are 30 to 900 carbon atoms in one molecule of a fullerene.
iii. Fullerenes are soluble in organic solvents such as carbon disulphide, chlorobenzene.
13. Give uses of allotropes of carbon : Fullerene
Ans Fullerene: The uses of Fullerenes are
a. Fullerenes are used as insulators.
b. Fullerenes are used as a catalyst in water purification.
c. At a certain temperature, fullerene exhibits superconductivity.
14. Give the properties: Covalent Compounds
Ans: The properties of covalent compounds are:
i. Covalent compounds have low melting and boiling points.
ii. They are generally insoluble in water and soluble in organic solvents.
iiii. .They are poor conductors of heat and electricity.
15. Give physical properties of Charcoal.
Ans 1. Charcoal is a light weight, black, amorphous form of carbon.
2. It is a highly porous and brittle material.
3. It is a bad conductor of heat and electricity.
4. It has low density.
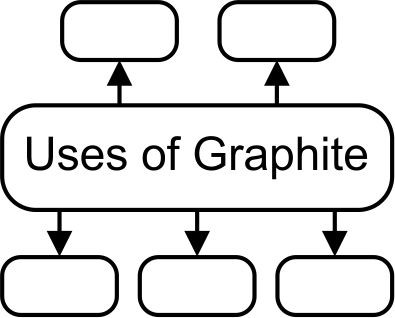
16. Give uses of allotropes of carbon : Graphite Ans Graphite: The uses of Graphite are
a. Graphite is used for making lubricants.
b. Graphite is used for making carbon electrodes.
c. Graphite is used in pencils for writing.
D. Graphite is used in paints and polish.
e. Graphite is used in arc lamps which give a very bright light.
17. Give uses of allotropes of carbon : Coke
Ans Coke: The uses of coke are:
a. Coke is used as domestic fuel.
b. Coke is used as a reducing agent.
c. Coke is used in production of water gas (CO+H2) and producer gas (CO+H2+CO2+N2)
18. Give practical uses of Carbon dioxide.
Ans: The uses of Carbon dioxide are:
i. Carbon dioxide is used to make aerated drinks.
ii. Solid carbon dioxide is used in cold storage and also to keep milk and milk products and frozen substances cool during transport. It is also used for getting special effects of a mist in dramas and movies.
iii. Liquified Carbon dioxide is used to remove caffeine from coffee.
iv. Liquid Carbon dioxide is used as solvent in modern eco-friendly dry cleaning.
v. Carbon dioxide obtained by chemical reaction or kept under pressure is used in fire extinguishers.
Q.11. Distingui sh between: 41.
form of carbon and Non-Crystalline form of carbon.
Ans:
| i. | A Crystalline form has a regular and definite arrangement of atoms. | A non-crystalline form does not have a regular and definite arrangement of atoms. |
| ii. | They have high melting and boiling points | They have low melting and boiling points. |
| iii. | Diamond, Graphite and fullerene are different crystalline forms of carbon | Coal, Charcoal and coke are the different non- crystalline/ amorphous form of carbon. |
| iv. | The crystalline form has a definite geometrical shape. | The non-crystalline form does not have a definite geometrical shape. |
2. Diamond and Graphite
Ans
| Diamond | Graphite | |
| i. | Diamond is brilliant, hard and crystalline allotrope of carbon. | Graphite is black, soft, brittle and slippery crystalline allotrope of carbon. |
| ii. | In diamonds, the carbon atoms forms a tetragonal three dimensional structure | In graphite, the carbon atoms forms a hexagonal layered structure |
| iii. | Diamond is a bad conductor of electricity. | Graphite is a good conductor of electricity. |
| iv. | Density of diamond is 3.5 g/cm3 | Density of graphite is 1.9 to 2.3 g/cm3 |
Q.12.Give scientific reasons : 16
1. Limewater turns milky when CO2 is passed through it.
Ans When carbon dioxide is passed through limewater, it turns milky white due to the formation of white, insoluble calcium carbonate (CaCO3) which precipitates out of the solution.
Ca(OH)2(aq) + CO2(g) → CaCO3(s) ↓ + H2O(l)
Limewater Carbon dioxide Calcium Carbonate Water
(White precipitate)
2. Why is a single bond between two carbon atoms strong and stable?
Ans i. Covalent bonds are formed by sharing of electrons between two atoms.
ii. A single covalent bond is formed when only one pair of electrons is shared between atoms.
iii. In this type of sharing, the atomic orbitals directly overlap between the nuclei of two atoms forming the strongest type of bond called the sigma bond.
iv. Hence a single covalent bond between two carbon atoms is strong and stable.
3. Biogas is an eco-friendly fuel.
Ans Biogas is formed by the anaerobic decomposition of animal dung, dry leaves, wet garbage etc. in a biogas plant to produce methane gas or biogas.
i. Biogas contains 55% to 60% of methane which burns completely without any smoke.
ii. Burning of animal dung, agricultural wastes and dry leaves would have resulted in the production of smoke and other green house gases.
iii. This will cause not only atmospheric pollution but also various respiratory disorders.
iv. Also the surroundings become clean and pathogens causing cholera, typhoid and jaundice are trapped as the wastes are decomposed in the biogas plant and not allowed to accumulate
v. Hence it is an eco-friendly gas.
4. Graphite is not used in ornaments.
Ans i. . Graphite is a crystalline allotropic form of carbon.
ii. It is a black, soft, brittle and dull form of carbon.
iii. It is neither malleable nor ductile.
iv. These properties of graphite make it unsuitable for the making of ornaments.
v. Hence, graphite is not used for making ornaments.
5. Graphite is a conductor of electricity.
Ans i. Graphite is a crystalline allotropic form of carbon.
ii. In Graphite, each carbon atom is bounded to three other carbon atom is such a way that a hexagonal layered structure is formed.
iii. Due to this structure, graphite has free electrons available.
iv. These free electrons move continuously within the entire layer.
v. Hence graphite is a good conductor of electricity.
6. Methane is called marsh gas.
Ans i. Methane gas is continuously formed by the decomposition of plants and animal matter in the absence of air, in swamps or marshy areas.
ii. So methane is found abundantly in marshy areas, and hence it is called as marsh gas.
7. Diamond is a non-conductor of electricity.
Ans i. A carbon atom has four valence electrons.
ii. In a diamond crystal, each carbon atom is linked to four other carbon atoms by covalent bonds.
iii. All four valence electrons of each carbon atom are used up in forming the bonds.
iv. Since there are ‘no free electrons in a diamond crystal, it does not conduct electricity.
8. Density of CO2 is more or less than that of air.
Ans i. The density of a substance is defined as mass per unit volume.
ii. At standard temperature and pressure, the density of air is 1.27Kg/m3 and the density of Carbon dioxide is 1.98 Kg/m3
iii. Hence the density of CO2 more than that of air.
Q.13. Give explanation using the given statement: 9
1. Lime water turns milky when CO2 is passed through it. Explain.
Ans i. . Lime water is a weak solution of the alkali Calcium hydroxide Ca(OH)2.
ii. When CO2 is passed through limewater it reacts with Calcium hydroxide to form insoluble precipitate of Calcium carbonate (CaCO3) ( weak basic salt)
iii. Calcium carbonate is white nd insoluble in water and hence remain suspended through out lime water.
iv. Chemical reaction:
CO2 + Ca(OH)2 ———–→ à CaCO3 + H2O
Carbon dioxide Calcium hydroxide Calcium carbonate Water
(lime water)
Hence, Lime water turns milky when CO2 gas is passed through it.
2. Petrol, diesel, coal are fossil fuels. Explain.
Ans i. . A fossil fuel is formed by natural processes, such as decomposition of buried dead organisms, existed in the ancient past. Fossil fuels contain high percentage of carbon.
ii. Coal is formed from the remains of trees and other vegetation. These remains were trapped at the bottom of the seas/ swamps and acted by the internal heat of the earth and extreme pressure of the sea water.
iii. Gradually the remains accumulated in the form of layers and created a dense material called peat. Peat is the first step in the formation of coal.
iv. Petrol and diesel are obtained from mineral oil. Mineral oil is also called as crude oil or petroleum oil is formed from plants and animals that lived in the seas millions of years ago.
v. Thus petrol, diesel and coal are called fossil fuels.
3. Diamond, Graphite and fullerenes are crystalline forms of carbon. Explain.
Ans i. Carbon shows the property of allotropy. Allotropy means existence of elements in more than one form having same chemical properties but different physical properties.
ii. Carbon exists in crystalline as well as non-crystalline form.
iii. The crystalline form has a regular and definite arrangement of atoms. They have high melting and boiling points. They have a definite geometrical shape, sharp edges and plane surfaces. Carbon has three crystalline allotropes such as Diamond, Graphite
and Fullerene.
iv. In the structure of diamond, every carbon atom is bonded to four neighboring atoms by covalent bonds. Therefore, diamond has a tetragonal three dimensional structure which makes it very hard.
v. In the structure of graphite, every carbon atom is bonded to three other carbon atoms in such a way that a hexagonal layered structure is formed.
vi. In fullerene, there are 30 to 900 carbon atoms in one molecule of a fullerene. The molecules of fullerene are found in the form of buckyballs and buckytubes. Fullerene is rarely found in nature.
Q.14. Attempt the following. 9
1. Reaction of Carbon with oxygen.
Ignite a coal on the burner and hold a moist blue litmus paper over the gas released on igniting the coal. Note the observation and answer the following questions.
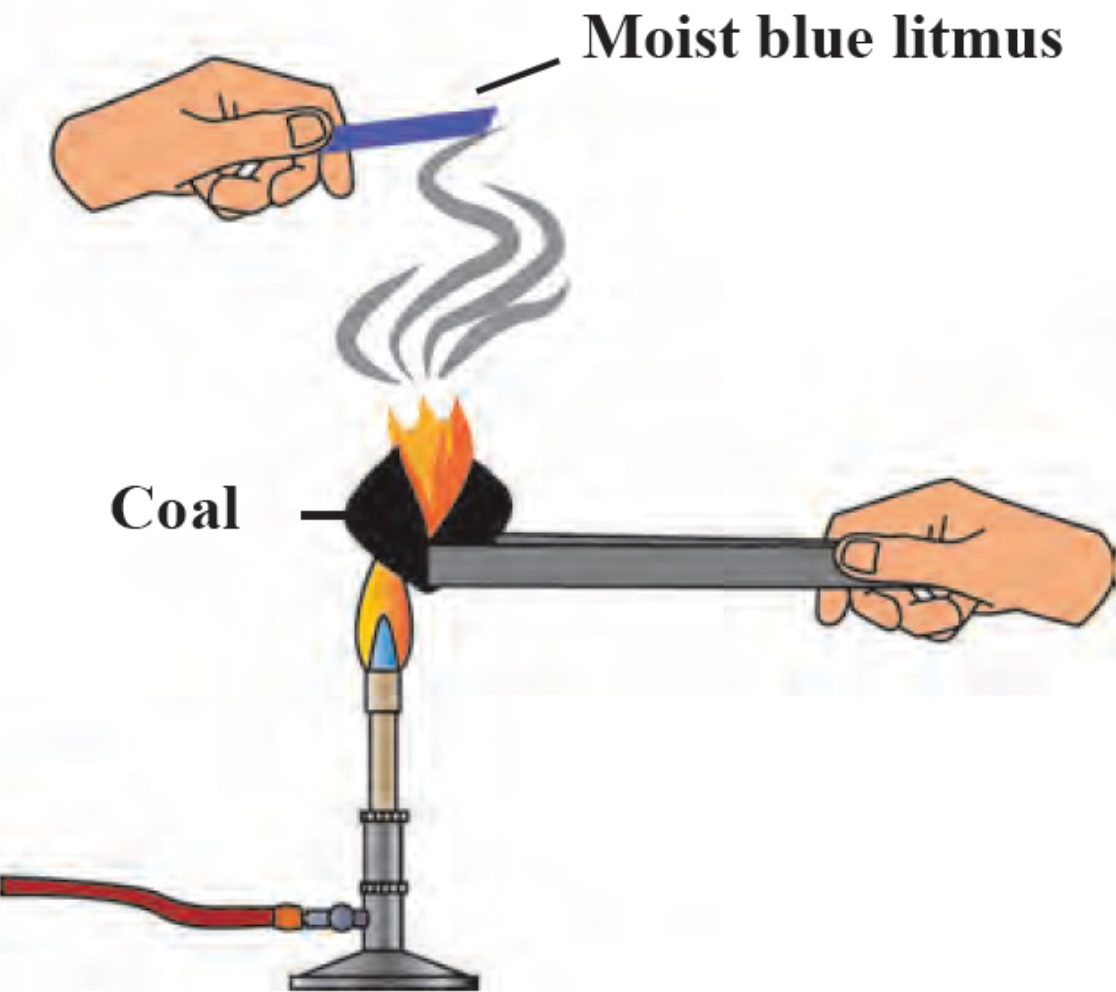
i. With which gas in the air does the coal react on igniting?
ii. What is the substance formed?
iii. What change takes place in the litmus paper?
iv. Write down the chemical reaction taking place in the above procedure.
Ans i. Coal reacts with the oxygen gas present in the air.
ii. The substance formed is Carbon dioxide.
iii. The moist blue litmus paper turns red.
iv. Carbon combines with oxygen to form Carbon dioxide.
Chemical reaction: C + O2 → CO2
Carbon Oxygen Carbon dioxide
2. A solid element X has four electrons in the outermost shell of its atom. An allotrope Y of this element is used as a dry lubricant in machinery and also in making pencil leads.
a. Name the element X and allotrope Y?
b. State whether allotrope Y is a good conductor or non-conductor of electricity.
C .Name two other allotrope of element X.
Ans i. The element is Carbon and the allotrope is Graphite.
ii. Y is a good conductor of electricity. iii. Diamond and Fullerene.
3. Label the following in the given diagram:
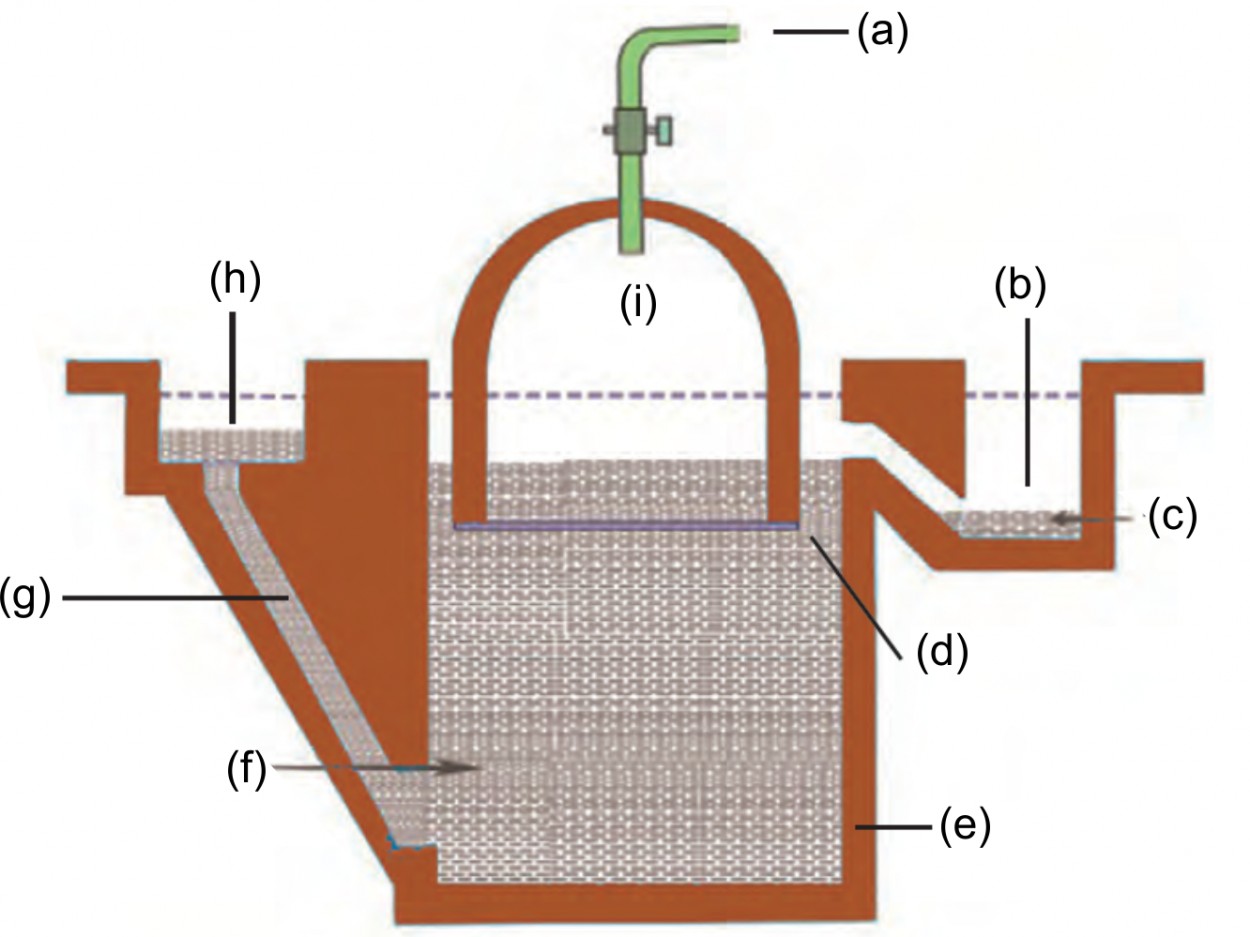 Biogas plant.
Biogas plant.
 Ans:
Ans:
Q.15. Complete the table/ web/ flow chart 9
1. Complete the following table regarding the information of Hydrocarbons.
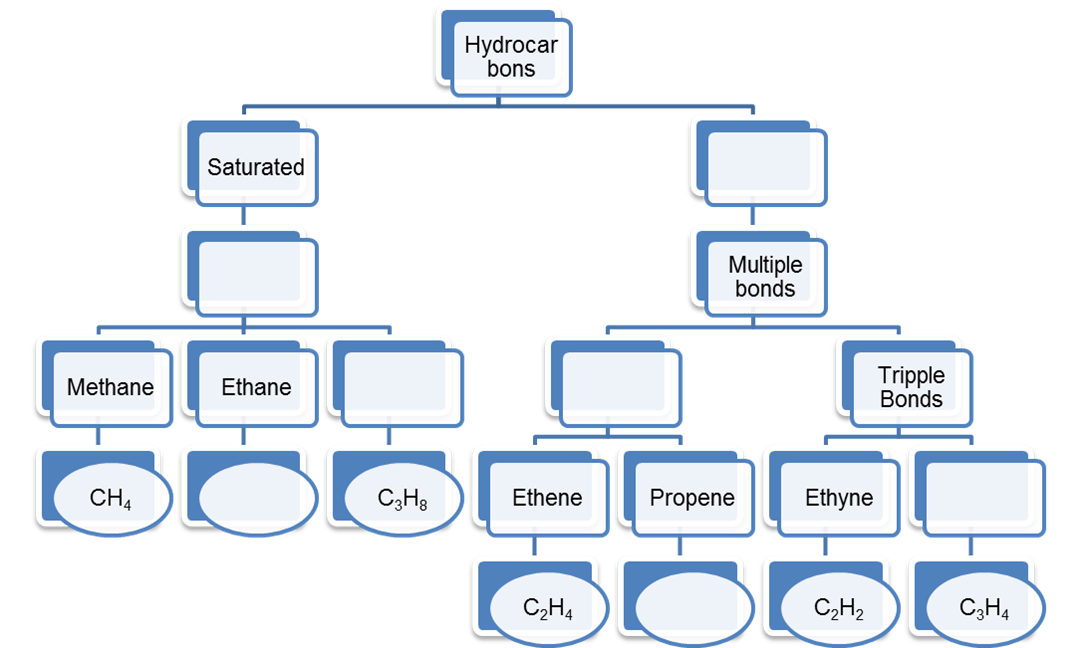
Ans:
2. Complete the following table regarding the information of Carbon.
| Symbol | Atomic Number | Atomic mass | Electronic Configuration | Valency | Metallic/Non-Metallic |
Ans Complete the following table regarding the information of Carbon.
| Symbol | Atomic Number | Atomic mass | Electronic Configuration | Valency | Metallic/Non-Metallic |
| C | 6 | 12 | (2, 4) | 4 | Non-Metallic |
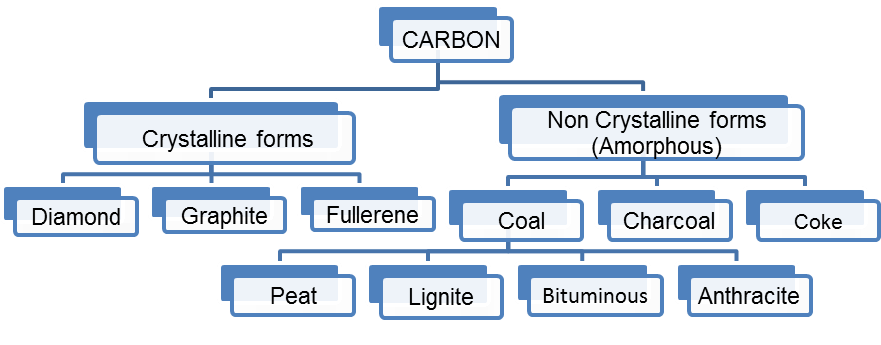
3. Classify Carbon
Ans
Q. 16. Complete the sentences in paragraph 9
1. Complete the paragraph:
(amorphous, crystalline, three, four, six, diamond, graphite, fullerene)
Allotropy is the phenomenon in which an element exists in different physical forms having different physical properties but similar chemical properties. Carbon occurs in several allotropic forms. Diamond, graphite and fullerenes are allotropes of carbon.In ……………, each carbon atom is bonded to neighbouring atoms by covalent bonds forming a tetragonal three-dimensionalstructure. In ……………, each carbon atom is bonded to neighbouring atoms by covalent bonds forming a hexagonal layered structure. molecules are found in the form of buckyballs and buckytubes.
Ans Allotropy is the phenomenon in which an element exists in different physical forms having different physical properties but similar chemical properties. Carbon occurs in several allotropic forms. Diamond, graphite and fullerenes are crystalline allotropes of carbon. In diamond, each carbon atom is bonded to four neighbouring atoms by covalent bonds forming a tetragonal three-dimensional structure. In graphite, each carbon atom is bonded to three neighbouring atoms by covalent bonds forming a hexagonal layered structure. Fullerene molecules are found in the form of buckyballs and buckytubes.
2. Complete the paragraph.
(55% to 60%, methanogenic, microbes, 65% to 70%, nitrogen, acetic, nitric, carbon dioxide, anaerobic)
Production of biogas is an …………………. process. It takes place in two stages. The act on the biodegradable complex organic compounds and produce organic acids such as …………………… acid. The bacteria act on the organic acids to produce methane gas which is also called biogas. Biogas contains about ……………………. methane and the rest is It is a fuel which is convenient to use and, in addition to this, a very good manure is also produced as a side product of the process.
Ans Production of biogas is an anaerobic process. It takes place in two stages. The microbes act on the biodegradable complex organic compounds and produce organic acids such as acetic acid. The methanogenic bacteria act on the organic acids to produce methane gas which is also called biogas. Biogas contains about 55% to 60% methane and the rest is carbon dioxide. It is a fuel which is convenient to use and, in addition to this, a very good manure is also produced as a side product of the process.
3. Complete the paragraph.
(nickel, 87%, marshy, 360°C, cobalt, 92%, Methane, 300°C, marsh)
………………. is the major component of natural gas, about by volume. Decomposition of organic matter in the absence
of air produces it. Hence, it is produced in a biogas production plant. It is found in coal mines. It is found at the surface of
…………………. places and hence it is also called as gas. On heating, a mixture of hydrogen and carbon monoxide
gases at ………………….. in the presence of , methane gas is formed. Fractional distillation of natural gas gives methane
in this pure form.
Ans : Methane is the major component of natural gas, about 87% by volume. Decomposition of organic matter in the absence of air produces it. Hence, it is produced in a biogas production plant. It is found in coal mines. It is found at the surface of marshy places and hence it is also called as marsh gas. On heating, a mixture of hydrogen and carbon monoxide gases at 300°C in the presence of nickel, methane gas is formed. Fractional distillation of natural gas gives methane in this pure form.
Q.17. Answer the following 27
1. Why are carbon and its compounds used as fuels?
Ans i. The name ‘carbon’ is derived from Latin word ‘carbo’ meaning coal. In the earth’s crust carbon is present to an extent of approximately 0.27% in the form of carbonate, coal, and petroleum.
ii. One of the non-crystalline and amorphous form of carbon is coal. Coal is a fossil fuel while charcoal and coke are the other amorphous forms of carbon used as fuel.
iii. Compounds of carbon such as hydrocarbons consist of carbon and hydrogen and are easily combustible. For example: Methane burns completely by reacting with oxygen in the air to form carbon dioxide and water.
iv. Thus, when hydrocarbons are burnt in air, large amount of heat is evolved with formation of carbon dioxide and water. Due to evolution of heat on combustion, carbon and its compounds are used as fuels.
2. How will you verify the properties of carbon dioxide?
Ans The properties of Carbon dioxide can be verified in the following ways.
i. When a burning candle is placed in a gas jar of carbon dioxide, it extinguishes indicating that Carbon dioxide is a non-combustible gas and does not support combustion.
ii. When Carbon dioxide gas is passed through lime water, it turns lime water milky due to the formation of insoluble Calcium carbonate.
iii. Moist blue litmus turns red in a gas jar of Carbon dioxide indicating that it is acidic in nature.
iv. Carbon dioxide is fairly soluble in water forming Carbonic acid.
3. State the properties of Graphite.
Ans: The properties of Graphite are:
i. Graphite found in nature is black, soft, brittle and slippery.
ii. Inside each layer of graphite, free electrons move continuously within the entire layer. That is why graphite is a good conductor of electricity.
iii. Due to the layered structure graphite can be used for writing on paper.
iv. The density of graphite is 1.9 to 2.3 g/cm3.
v. Graphite does not dissolve in most solvents.
4. What is an element? What the different types of element?
Ans i. A substance which cannot be decomposed by simple substances by any physical or simple chemical methods is called as an element.
ii. An element is composed of atoms of only one kind. iii. The different types of elements are:
a. Metals: example- Sodium, Calcium, Gold, etc.
b. Non-Metals: example – Oxygen, Sulphur, Carbon, etc.
c. Metalloids: example – Silicon, Antimony, etc.
5. What is a compound? How are compounds formed?
Ans i. A compound is formed when two or more elements chemically combine with each other in definite proportion by weight.
ii. The properties of a compound are altogether different from its constituent elements.
iii. For example: Pure water is made up of two elements Hydrogen and Oxygen. These elements chemically react with each other in a definite proportion of (1:8) by weight to form water. The properties of water are altogether different from properties of hydrogen and oxygen.
6. In which compound form does carbon occur?
Ans Carbon in its combined state exists in form of various compounds such as:
i. Carbon dioxide and in the form of carbonates such as Calcium carbonate, marble, calamine (ZnCO3)
ii. Fossil fuels-coal, petroleum, natural gas.
iii. Carbonaceous nutrients- carbohydrates, proteins, fats.
iv. Natural fibres-cotton, wool, silk.
v. Hydrocarbons- Compounds containing carbon and hydrogen.
7. Explain the structure of diamond and graphite?
Ans i. Diamond : In diamond, every carbon atom is bonded to four neighbouring atoms by covalent bonds. Therefore, diamond has a tetragonal three dimensional structure which makes it very hard.
ii. Graphite : Every carbon atom in graphite is bonded to three carbon atoms in such a way that a hexagonal layered structure is formed. A graphite crystal is made up of many sheets or layers of carbon atoms. These layers slip over each other on applying pressure. One layer of graphite is called graphene.
8. What type of an element is carbon? Give some information about it.
Ans i. Carbon is a non-metallic element.
ii. It is available abundantly in nature and occurs in free as well as combined state. Iii .Carbon has an atomic number of 6 with an electronic configuration of (2,4)
iv.It is tetravalent as it has four valence electrons.
v.It forms covalent bonds.
9. I. Does an electric charge form on atoms when a covalent bond is formed between them?
ii. . What are organic and inorganic compound?
Ans i. No, an electric charge is not formed on atoms when a covalent bond is formed between them.
This is because, covalent bond is formed by sharing of electrons and so there is no change in number of electrons and protons in these atoms and they remain electrically neutral.
ii. . Compounds obtained directly or indirectly from plants and animals are called Organic compounds. Compounds obtained from minerals are called inorganic compounds.
Q.18. Extra data (Not to be Use) 5
1. State the properties and uses of Graphite.
Ans The properties of Graphite are:
i. Graphite found in nature is black, soft, brittle and slippery.
ii. Inside each layer of graphite, free electrons move continuously within the entire layer. That is why graphite is a good conductor of electricity.
iii. Due to the layered structure graphite can be used for writing on paper.
iv. The density of graphite is 1.9 to 2.3 g/cm3.
v. Graphite does not dissolve in most solvents.
The uses of Graphite are:
i. Graphite is used for making lubricants.
ii. Graphite is used for making carbon electrodes.
iii. Graphite is used in pencils for writing.
iv. Graphite is used in paints and polish.
v. Graphite is used in arc lamps which give a very bright light.
Q.19. Answer the following in detail 20
1. State the properties and uses of Methane
Ans The properties of Methane are:
i. Methane is a colorless gas.
ii. Methane is in gaseous state at room temperature.
iii. Methane is sparingly soluble in water. It is highly soluble in organic solvents like gasoline, ether and alcohol.
iv. Melting point of Methane is (-182°C)
v. Boiling point of Methane is (-161.5°C)
The uses of Methane are:
i. Methane in the form of natural gas is used in industries such as fabric mills, paper mills, food processing industry, petrol purification.
ii. Being the smallest hydrocarbon, the proportion of CO2 released in the combustion of methane is small and, therefore, it is used as a domestic fuel.
iii. Methane is used for production of organic compounds such as ethanol, methyl chloride, methylene chloride and acetylene.
2. Explain the properties of carbon.
Ans Allotropic nature of Carbon: Carbon shows the property of allotropy. Allotropy means existence of elements in more than one form having same chemical properties but different physical properties. The two allotropic forms of carbon are:
i. Crystalline Form : Carbon has three crystalline allotropes: Diamond, Graphite and Fullerene. The Properties of Crystalline forms of carbon are as follows:
a. A crystalline form has a regular and definite arrangement of atoms.
b. They have high melting and boiling point.
c. A crystalline form has a definite geometrical shape, sharp edges and plane surfaces.
ii. Non-crystalline Form : Coal, coke and charcoal are the non-crystalline forms of carbon. The properties of non-crystalline forms of carbon are as follows:
a. The arrangement of carbon atoms in this form is not regular.
B .They have a low melting and boiling points.
c. Most of them are used as fuels.
3. State uses and physical and chemical properties of Carbon dioxide.
Ans: The physical properties of Carbon dioxide are:
i. Carbon dioxide is an odorless gas.
ii. Carbon dioxide is a colorless gas.
iii. It is heavier than air.
iv. It is fairly soluble in water.
The chemical properties of Carbon dioxide are:
i. Carbon dioxide is non-combustible and does not support combustion.
ii.Carbon dioxide turns lime water milky.
iii. Carbon dioxide is fairly soluble in water and dissolves in water forming carbonic acid.
iv. Carbon dioxide turns blue litmus red indicating that it is acidic in nature.
v. The color of universal indicator turns orange/yellow in CO2. (pH value between 4 to 6)
The uses of carbon dioxide are:
i. Carbon dioxide is used to make aerated drinks.
ii. Solid carbon dioxide is used in cold storage and also to keep milk and milk products and frozen substances cool during transport. It is also used for getting special effects of a mist in dramas and movies.
iii. Liquified Carbon dioxide is used to remove caffeine from coffee.
iv. Liquid Carbon dioxide is used as solvent in modern eco-friendly dry cleaning.
v. Plants use Carbon dioxide in air for photosynthesis.
vi. Carbon dioxide obtained by chemical reaction or kept under pressure is used in fire extinguishers.
4. How will you prove experimentally that graphite is good conductor of electricity?
Ans Aim: To prove that graphite is a good conductor of electricity.
Apparatus: Lead pencil, electrical wires, battery/cell, small bulb.
Procedure:
i. Remove the lead from the pencil and assemble the apparatus as shown in the diagram.
ii. Start the electric current in the circuit and observe.
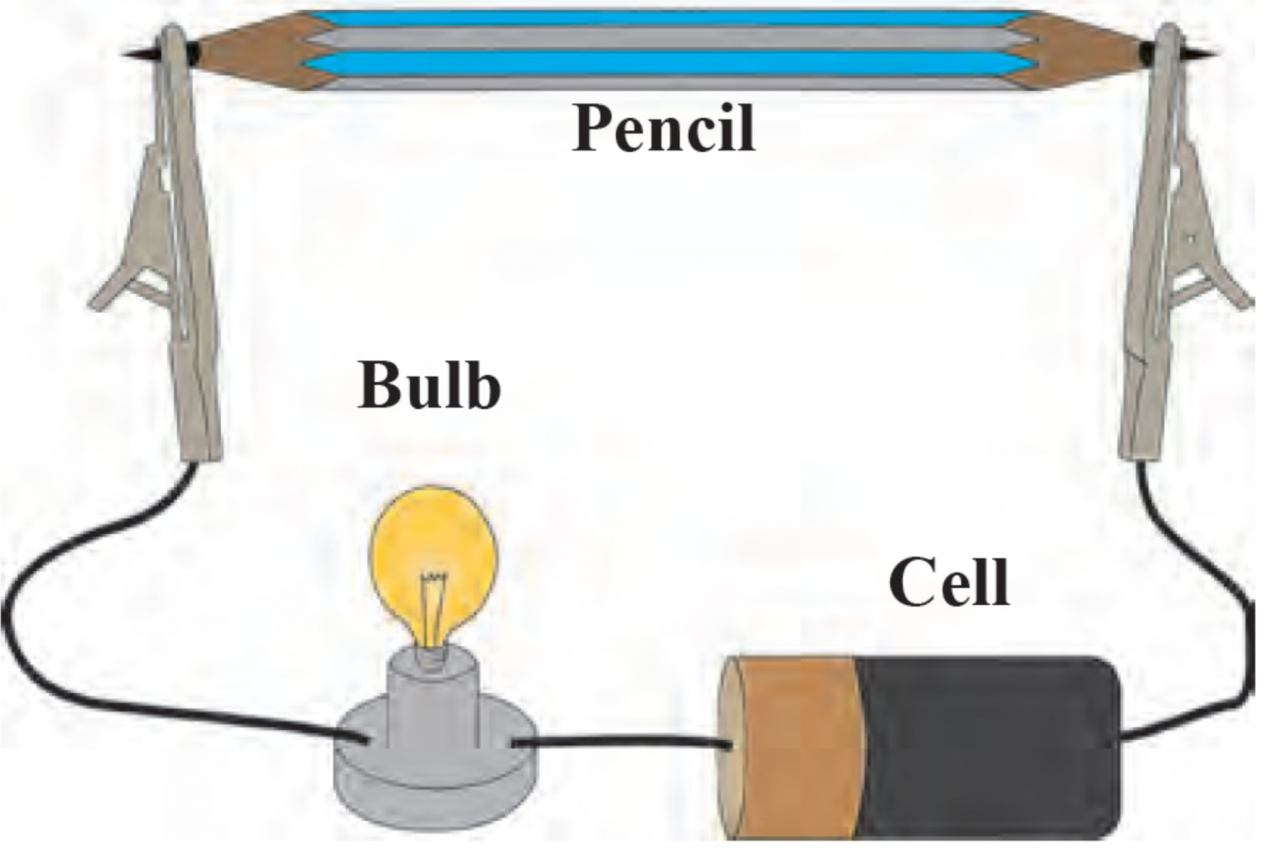
Observation: The moment the electric current is passed through the circuit, the bulb glows.
Conclusion: This experiment proves that graphite is a good conductor of electricity as graphite has free electrons that move continuously within the entire layer and conduct electricity in the lead of the pencil.